Please select the object link below for more information on how to configure the object.
Object |
Shared with other SmartSolve© Applications |
Required forTM SmartComplaints |
|
Yes |
Yes |
|
|
No |
Yes |
|
|
No |
Yes |
|
|
No |
Yes |
|
|
No |
Yes |
|
|
No |
Yes |
The European Union (EU) is moving towards supporting an electronic format for vigilance reports. SmartComplaintsTM supports the latest version of the MedDEV pdf report and the xml file necessary for submission to the competent authorities.
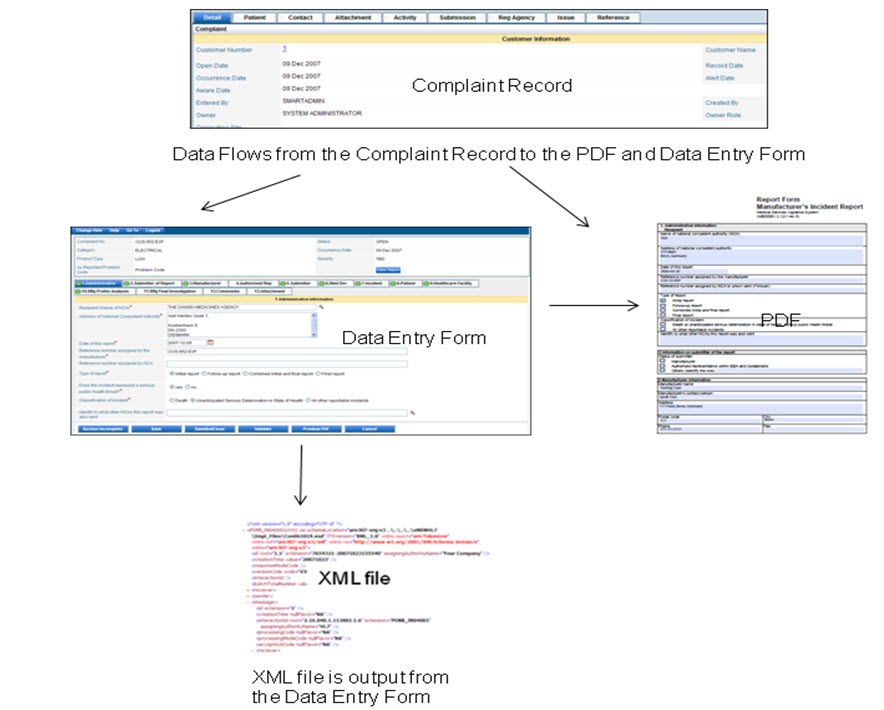
The following diagram shows how information flows from the manufacturer to the European Union Regulatory Agency.

The manufacturer:
1. receives a complaint.
2. creates the complaint record.
3. determines if the event is reportable.
4. completes report information
5. submits a manual pdf or an electronic xml file to the official regulatory body
Term |
Description |
|
eMDV |
Electronic MedDEV report (XML file format)
|
|
EU |
European Union
|
|
MedDEV |
Report currently accepted by the EU Competent Authorities and Notified Bodies
|
Please see Numbering Scheme under System Wide Setup.
Please see Submission Status under Manual Reporting Setup.
The Regulatory Reporting Policy is used for eMDV to:
· Enable eMDV submission capability.
· Define the MDV submission numbering scheme.
· Define the eMDV xml output file naming convention and directory location.
Please see Regulatory Reporting Policy under Manual Reporting Setup
Please see How to Access the PDF on the Server under Manual Reporting Setup.
Please see How to Map Fields from the Complaint to the PDF under Manual Reporting Setup.
You can configure your company name to populate into all the MedDEV PDF reports by setting up the following:
1. Access the folder - Application Name\SubmissionForms\ EU-QR-P12-01MEDFORM2.XSL
2. Change Company Name to your company name.

The HtmlXSLT=” EU-QR-P12-01MEDFORM2.XSL” file is used to map the data fields from the complaint record to the data entry template.
The table below shows the folders that have been added to support the eMDV feature that IT departments may need to access during the implementation stage.
Folder |
Description |
|
EUERRORFILE |
If there is an error when completing the submission, the system creates an error that is displayed on the user's screen and an xml file is created in this folder. The xml file name is the same as the complaint number. |
|
|
NOTE: Do not edit or remove this folder.
|
|
EUXML |
Default Location for the EU xml file.
|
|
|
NOTE: The system can be configured to export the file to other locations.
|
The eMDV xml file is copied to the DefaultPath= EUXML folder when eSubmission is selected in the Submission tab of the complaint record.
EUXML is the folder for the output of the xml default folder. The transport mechanisms need access to this directory.
Please see the Regulatory Reporting Policy under Manual Reporting Setup to configure the default Location
DefaultFormat = “{0}-MDV-Initial.xml" is the default naming convention used for all eMDV xml files that are created and by default stored in the EUXML file folder. This default file naming convention can be overwritten in the unique Regulatory Reporting Policy created for each registration site. This is the default applied to all the eMDV xml files.
Application Name\SubmissionForm\FormConfig.xml

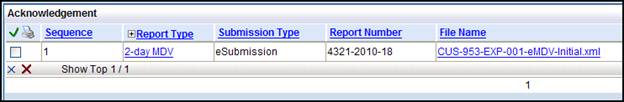
In the Submission tab look for the Submission Method column. Once you have submitted the eMDV an Acknowledgement hyperlink appears. Select the hyperlink to show you the file. You can check here quickly to see the immediate impact if you change the name.
Please see Forms Designer for more information on configuring the MedDEV HTML Form.
Please see How to Map Fields from Complaints to the HTML under eMDR Reporting Setup.
All verified information can be configured to reduce wait time. All fields are validated out of the box and the administrator can configure fields to eliminate checks that are not required.
The system validation capability must be configurable by the Administrator, if other mistake proofing measures are taken the administrator should be able to reduce the items in the file for validation; thereby reducing their end-users wait time in the system. A validation "base" is available to validate all the data entered in the html form against.
All information that is verified is configurable.
All fields will be validated out of the box and customer configuration should be to eliminate checks that are not required.